(Scroll)

Scriptera is a regulated pharmaceutical analytics platform designed to help pharmacies and industry partners interpret complex market data with clarity and accountability. Before Scriptera, insight lived across spreadsheets, exported reports, and disconnected tools. Interpretation varied by role. Authority was implied rather than enforced. In regulated environments, that gap creates real operational and compliance risk.
This engagement focused on designing a greenfield platform where data access, permissions, and accountability were structurally aligned. The result was a system where insight could be acted on with confidence, without ambiguity, and without compromising trust.
This engagement focused on designing a greenfield platform where data access, permissions, and accountability were structurally aligned. The result was a system where insight could be acted on with confidence, without ambiguity, and without compromising trust.
Client:
Headquarters:
Agency:
Industry:
Engagement:
Role:
Pharmaceutical organizations do not lack information. They lack systems that align insight with responsibility. Scriptera works with de-identified, market-level data to enable pattern recognition and compliance analysis without exposing patient information. Anonymization alone, however, does not remove risk. Interpretation still carries consequences when ownership is unclear.
As a zero-cost partnership platform, Scriptera earns trust structurally. Pharmacies retain control of their data, while value flows back to the organizations generating it. This required a system designed for judgment over speed.
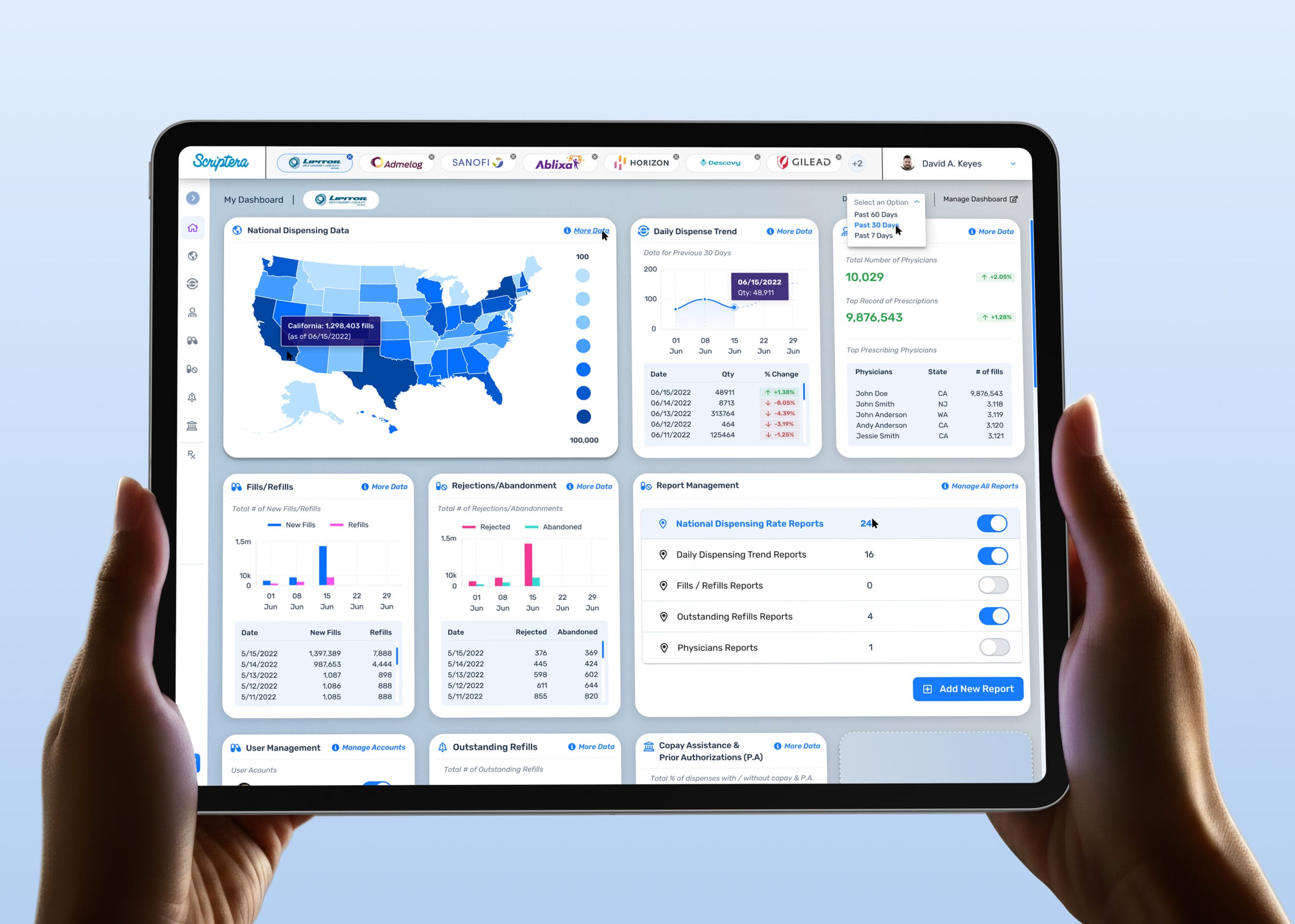

The problem was not complexity. It was false confidence.
Systems that surface insight without clear ownership encourage premature conclusions and ungoverned action. In regulated environments, that failure mode is subtle and dangerous.
The platform needed to ensure insight followed authority, decisions were traceable to accountable roles, and governance was embedded directly into workflows rather than enforced externally.
The guiding principle was simple. When responsibility is not clarified by the system, risk fills the gap.
Rather than compressing complexity into dense dashboards, the strategy focused on sequencing understanding. Data appeared with context. Permissions established authority. Reporting preserved intent as information moved between roles.
Customization existed within guardrails. Flexibility supported responsibility rather than undermining it. Governance was treated as a design requirement, not an operational afterthought. The objective was restraint. The system needed to hold up under regulatory scrutiny and real-world decision pressure.
Execution translated the product strategy into structural decisions. Early concepts were pressure-tested against data density, role boundaries, and regulatory constraints. Only patterns that preserved clarity and accountability advanced.
Every interface decision was evaluated against a single question: Does this support judgment under regulatory pressure?
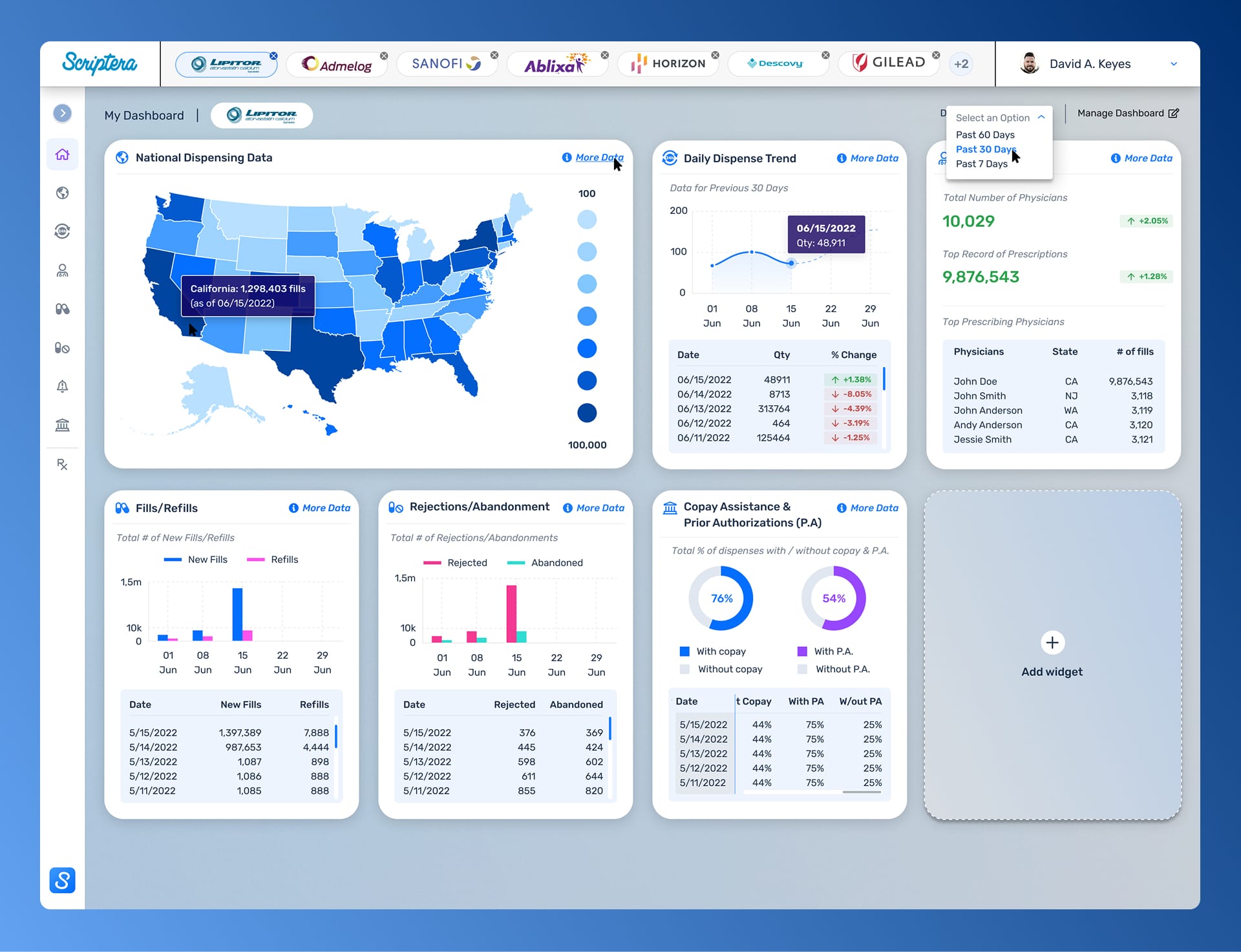
Dashboards were designed as modular systems rather than fixed views. Each widget functioned as an accountable data source, surfaced or suppressed by role without compromising system integrity.
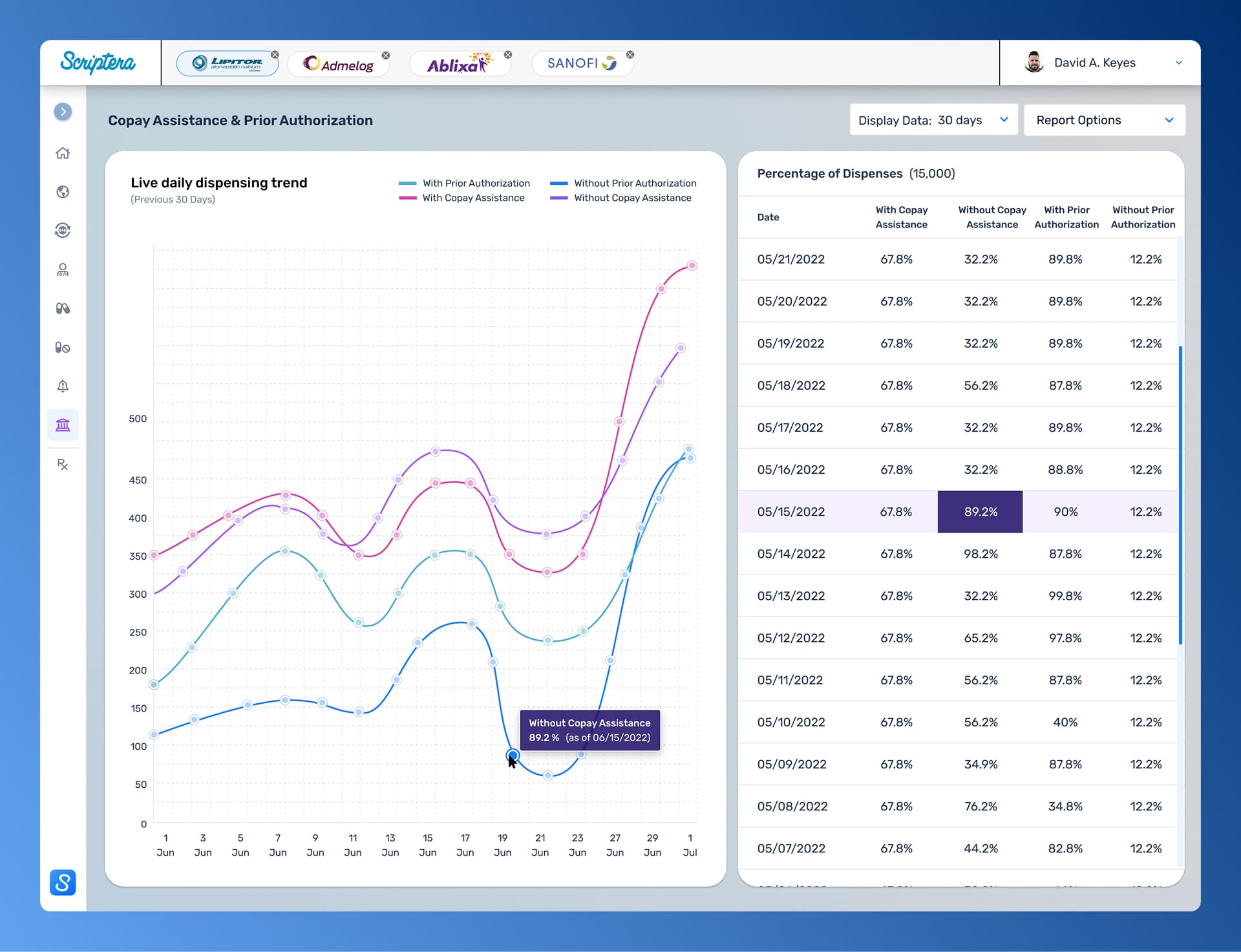
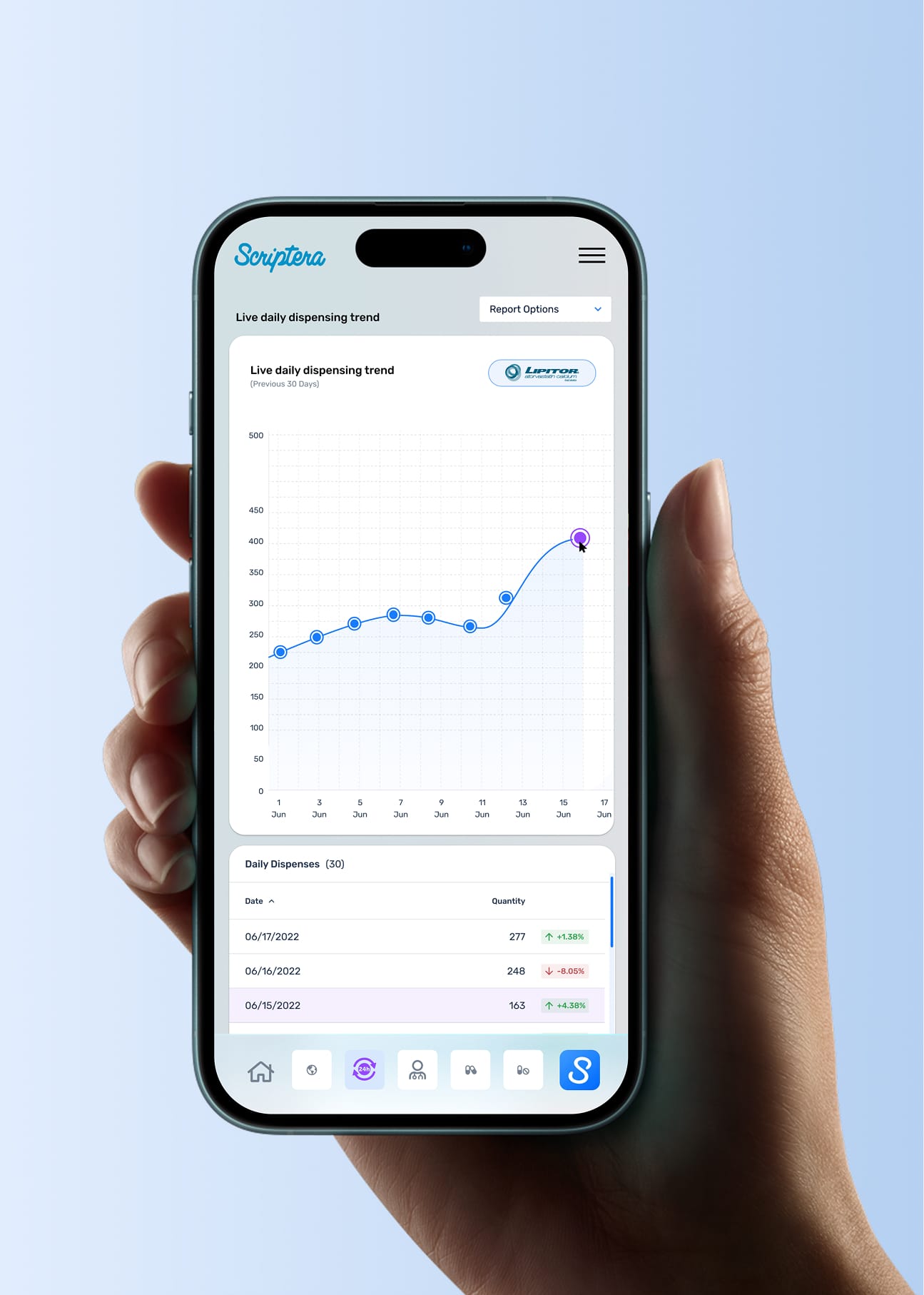
High-level signals were never treated as conclusions. Each dashboard surface is connected to deeper, role-aware views, sequencing information to support deliberate analysis rather than immediate reaction.
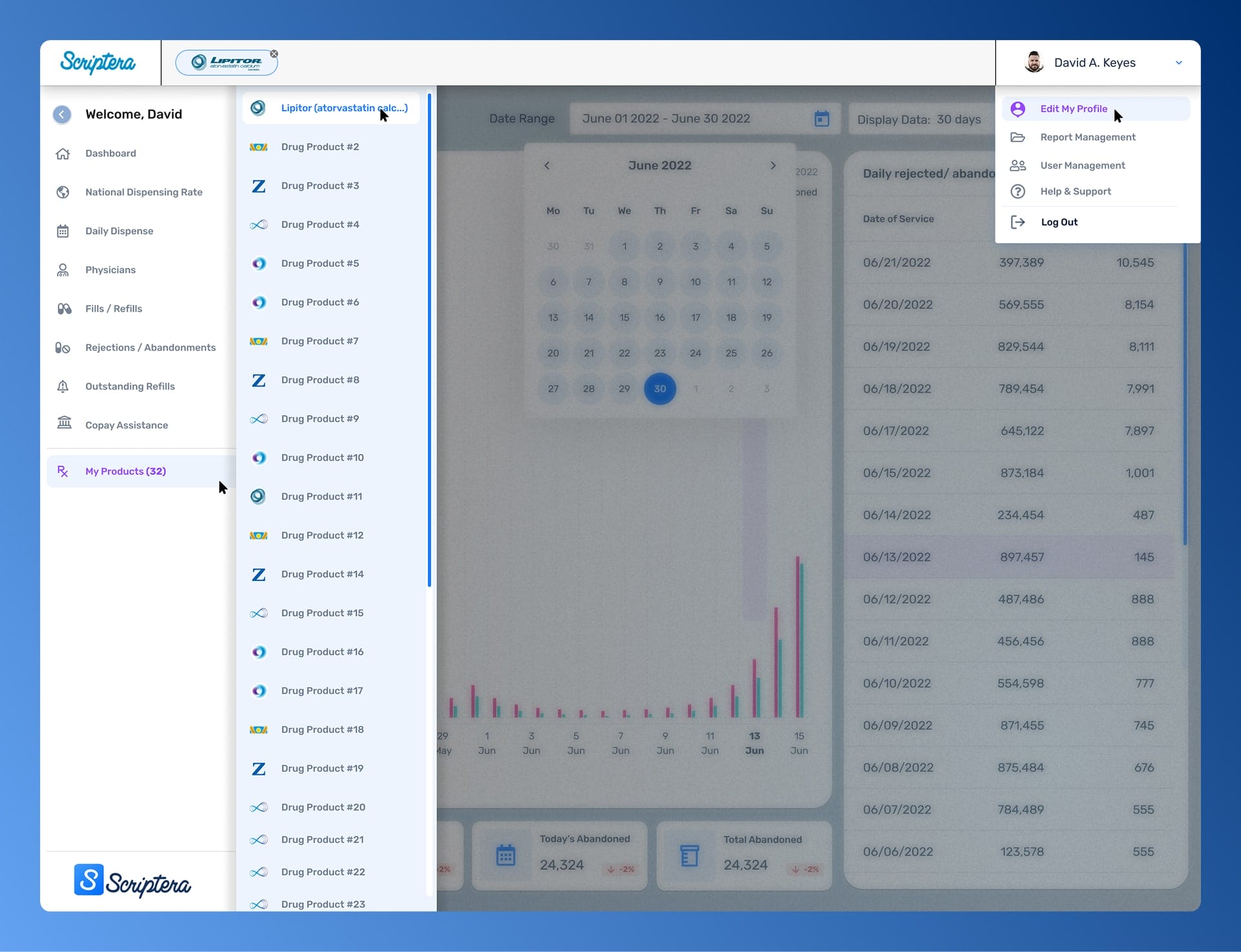
Supporting mechanics reinforced core decisions across the platform. Collapsible navigation managed data density, while governed reporting workflows preserved context and intent beyond the interface.
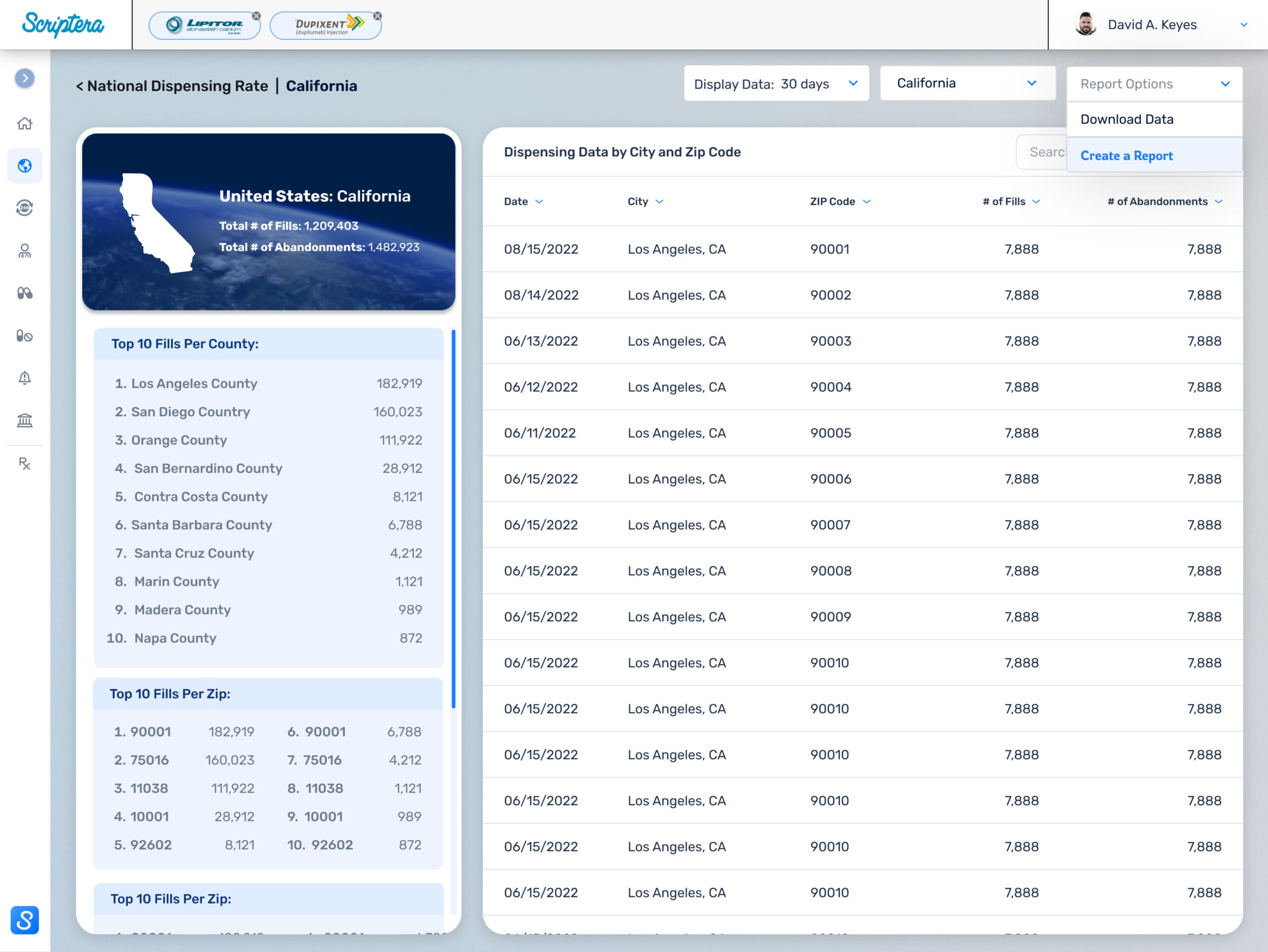

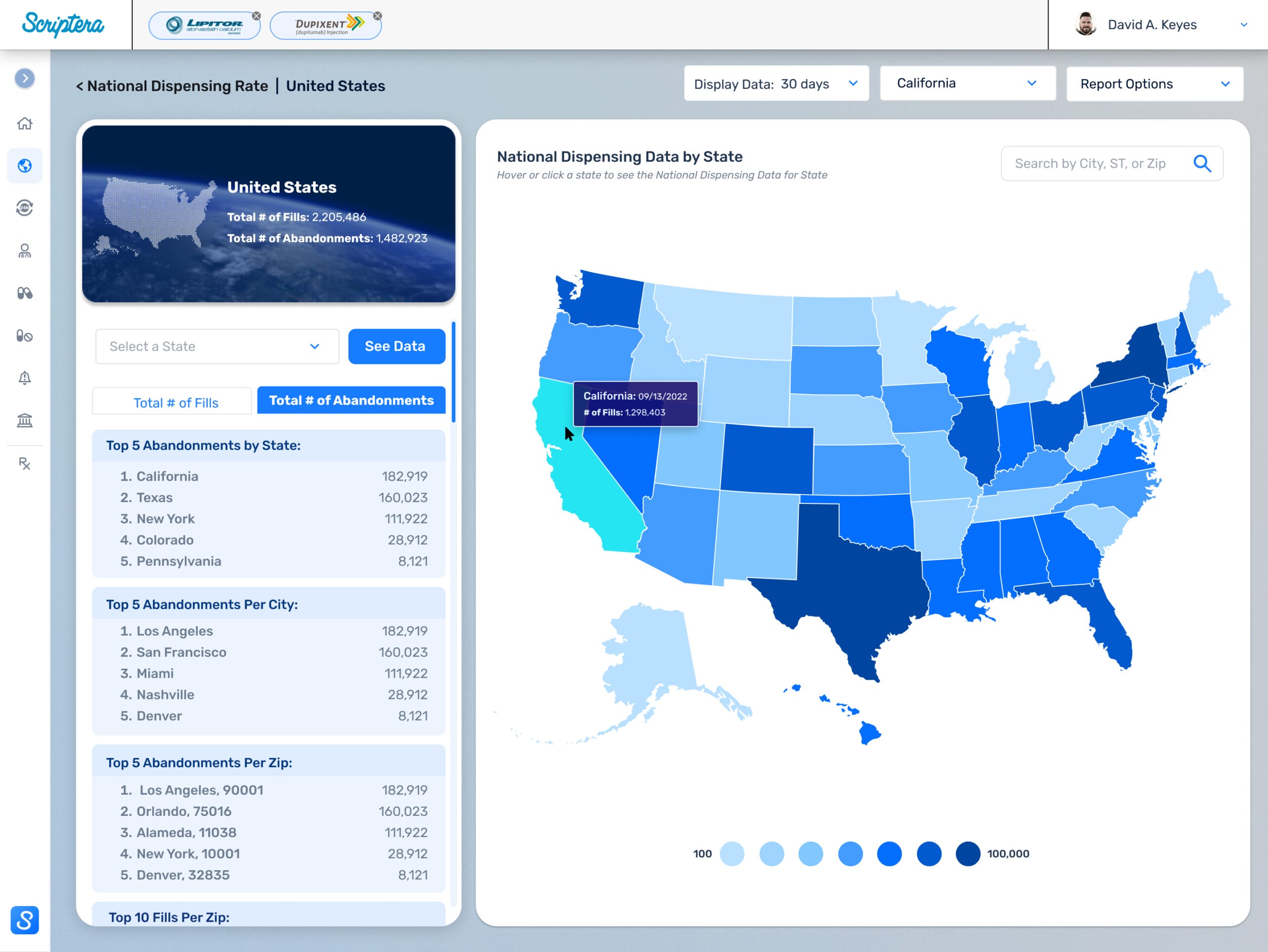
Delivered under a compressed timeline, the platform required early, decisive direction. I led product vision, UX strategy, and system architecture, mapping more than 75 role-specific screens and system states across administrators, compliance stakeholders, and general users.
Design intent was translated into development-ready requirements. UX execution was developed in collaboration with a UX designer under my creative and product direction at Saritasa.
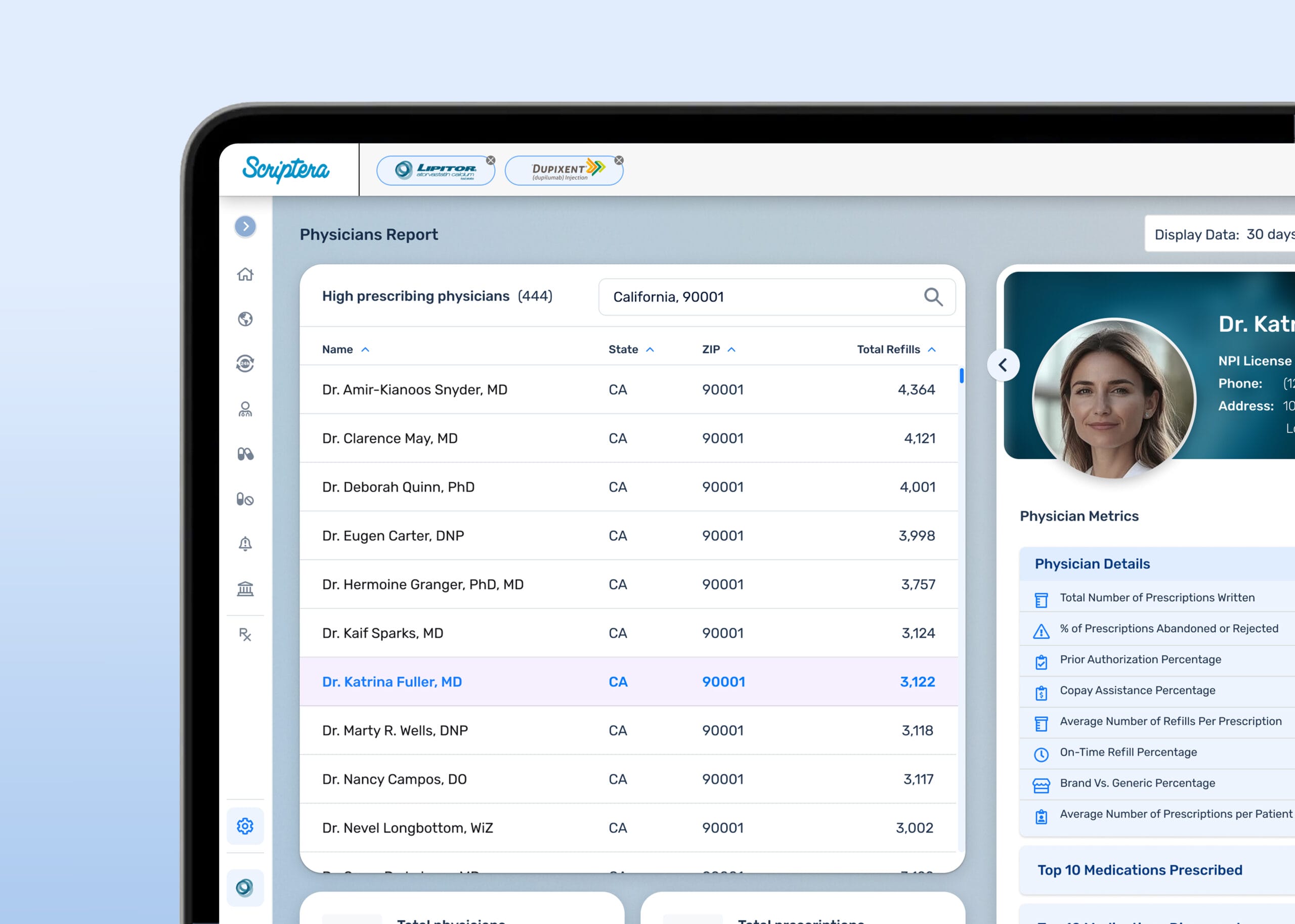

Teams spent less time reconciling reports and more time understanding them. Data sharing became intentional. Compliance shifted from procedural to structural.
Today, Scriptera supports data partnerships across thousands of retail pharmacies. The platform delivers structured insights and revenue opportunities at no cost, while allowing owners to retain value from their own data.


Scriptera was built for environments where accountability cannot be assumed or deferred. By aligning data access, permissions, and responsibility at the system level, the platform created a foundation for insight that scales without introducing regulatory or operational risk. Systems fail not from missing data, but from unclear ownership of the decisions data enables.
Regulated data platforms often fail not from missing information, but from unclear ownership of interpretation and action. This work shows how system design can enforce accountability by aligning data access, permissions, and responsibility at a structural level.
Work shown is presented for portfolio purposes; rights and trademarks belong to their respective owners, and some deliverables were created in collaboration with other parties or agencies. Please see site Terms of Use Policy for additional information.